Live device data ingestion
Readings flow into the patient record with timestamps and source tracking
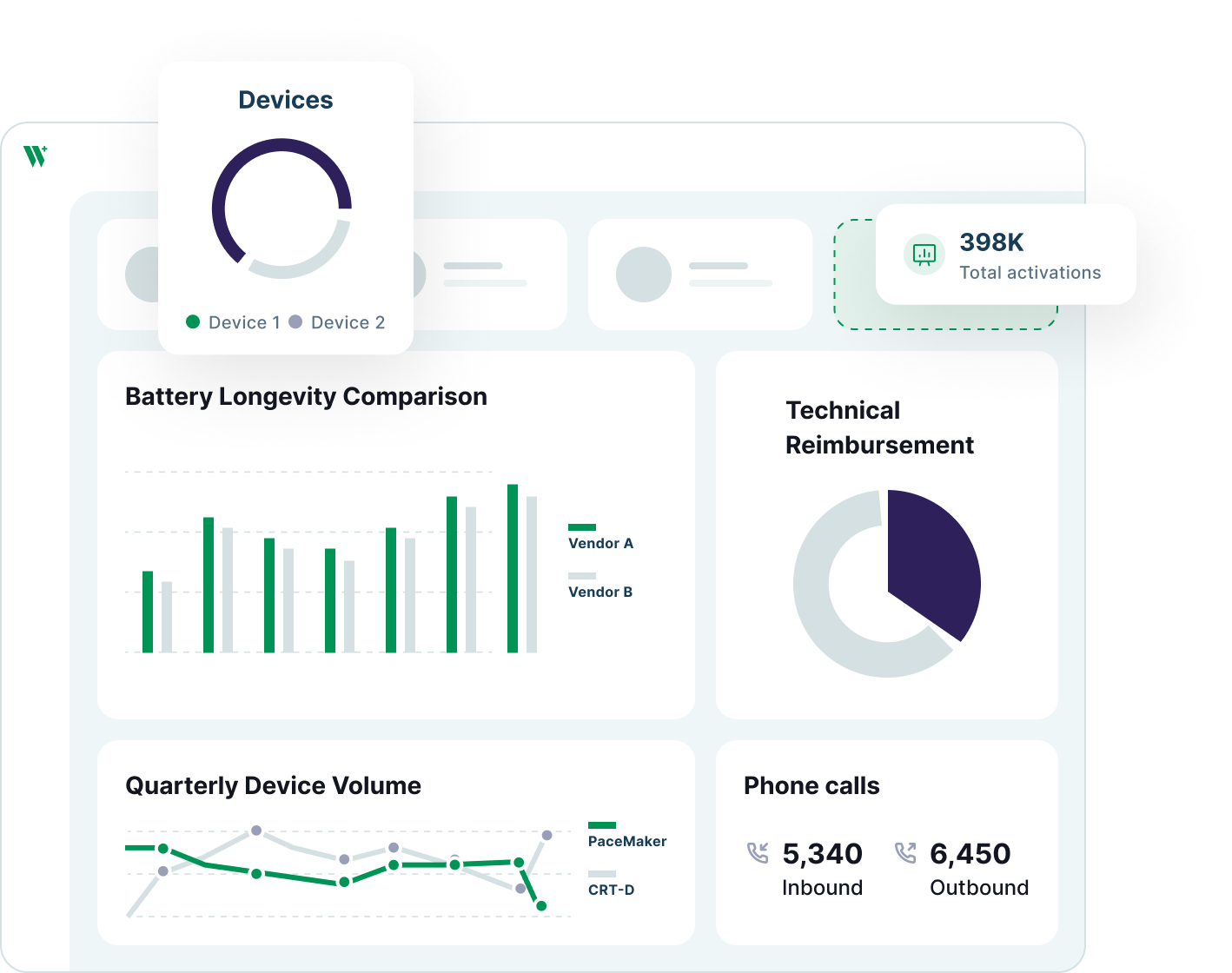
Welkin’s care management platform helps implantable medical device companies deliver scalable, compliant post-implant programs. Monitor device telemetry, automate care protocols, and generate real-world evidence
Device companies running post-implant programs across many sites. Clinical ops, patient services, and commercial teams that need speed, evidence, and audit-ready processes.
The fastest-growing
device companies use Welkin

Even with a strong team, an internal system isn’t “done” after v1
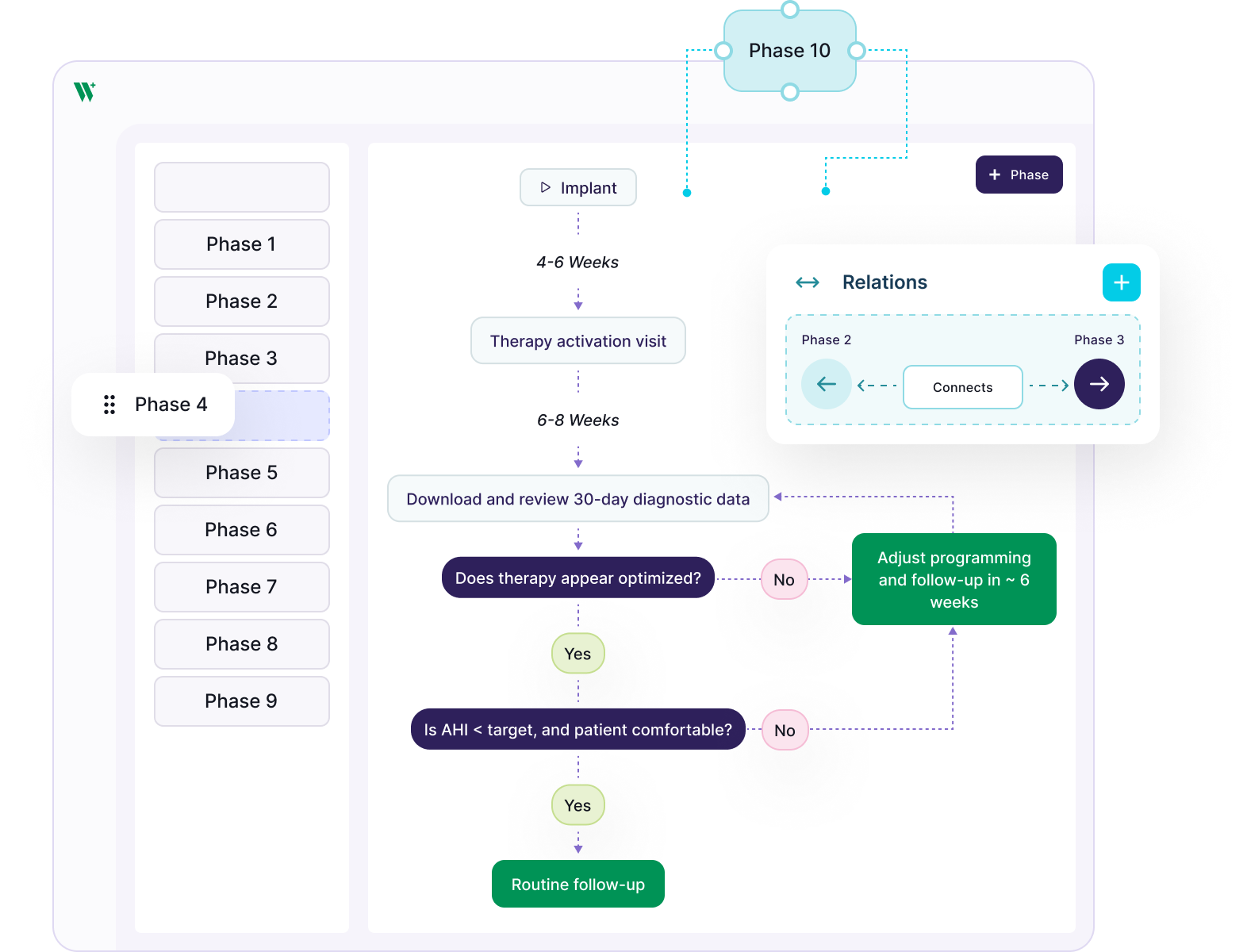
We don’t prescribe your workflow. You bring SOPs; we configure:
Prove that your device works – and use it to grow